About Us
In 2023, leadership of the MSGERC and ECMM convened an initial working group to explore clinical trial outcome criteria for clinical mycology (Aspergillus/Molds, Candida, Endemic/Dimorphic, and Cryptococcosis. The intent of the group was to convene senior leadership from ECMM and MSGERC (current and past presidents) to vision the structure and function of the group to ensure equality and inclusiveness across groups and to embrace early career investigators. The Executive, Steering and Disease Subgroup chairs are listed below. The disease subgroup chairs selected membership representation from each group. Their work is highlighted in those individual pages.
Unique to this endeavor is the transdisciplinary and multi-institutional and international nature of the consensus working group and the consensus agreement formed by the Executive Committee.
Executive Committee
MSGERC |
ECMM |
| Luis Ostrosky | Neil Gow |
| John Perfect | Martin Hoenigl |
| Peter Pappas | Oliver Cornely |
Steering Committee
MSGERC |
ECMM |
| Luis Ostrosky | Neil Gow |
| John Perfect | Martin Hoenigl |
| Peter Pappas | Oliver Cornely |
| George R Thompson | Johan Maertens |
| Dimitrios Kontoyiannis | Jean Pierre Gangneux |
| Monica Slavin | Connie Lass-Florl |
| Marisa Miceli | Patricia Munoz |
Disease Subgroups
Aspergillus/Other Moulds
Co-Chairs: Monica Slavin & Martin Hoenigl
Candida
Co-Chairs: Joe Vazquez & Sevtap Arikan
Cocci/Other Endemics
Co-Chairs: GR Thompson & Ana Alastruey Izquierdo
Cryptococcosis
Co-Chairs: David Boulware & Tihana Bicanic
Consensus Agreement
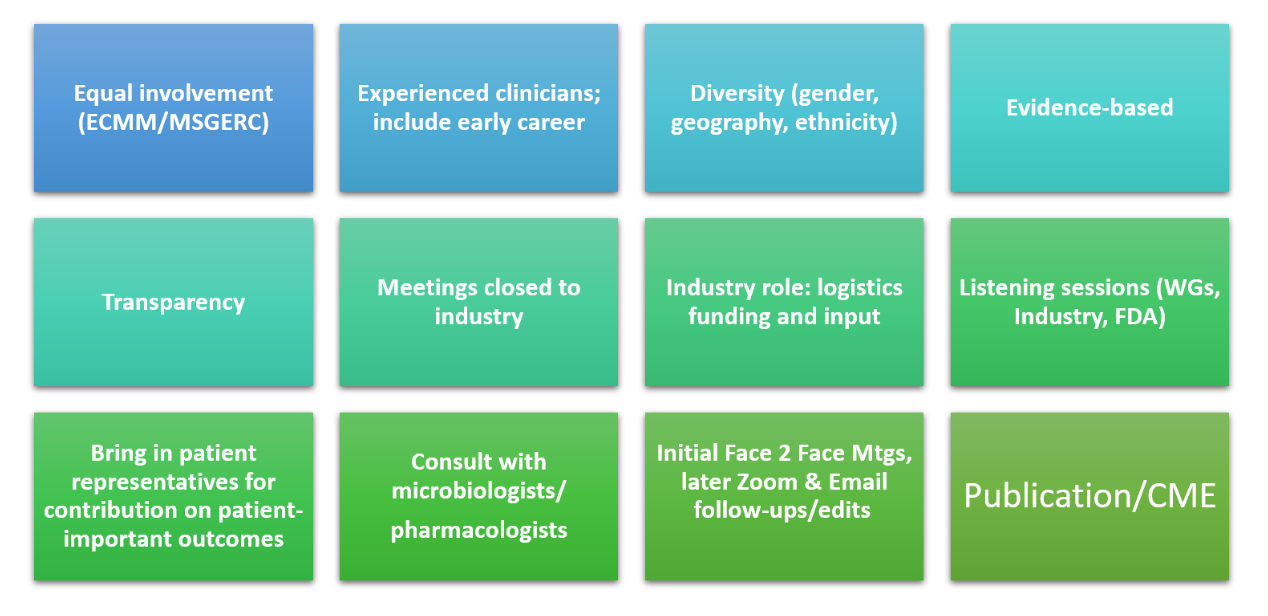
The Executive Committee and Steering Committee agree that there will be equal involvement (MSGERC and ECMM) and transparency of all activities. There will be a consensus approach for methodology in evaluating current response criteria, taking an evidence approach, and share collective experiences as investigators and as clinical trial Data Review Committee (DRC) and Data Safety Monitoring Board (DSMB). The groups are committed to diversity of committee and working group membership: geographic, gender and other criteria. The group will include early career investigators, but rely on experienced clinicians to guide processes to bridge earlier criteria to current need. Each working group will include at least one patient representative at some stage of the process. The groups will host listening sessions that will include investigators, patient representatives, industry, EMA, FDA and CDC. In terms of industry involvement, the process will take a pan-industry approach and working group sessions will be closed, not including industry representative to ensure an unbiased approach. The groups will include outreach to regulatory authorities (FDA and EMA) in addition to inviting them to listening sessions. Working groups are encouraged to meet face-to-face; however, some meetings may take place using Zoom and E-mail exchanges. Some meetings may take place at larger International Meetings and external funding from sponsors will be sought to help defray those costs. Committee and Sub-Group members agree that this is an activity that will not be reimbursing participants for the work of the committees and working groups and that the ECMM and MSGERC will share in-kind expenses. The MSGERC Headquarters will lead the scheduling and travel arrangements for meetings and some of the expensis for this will come from funds raised.